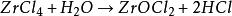Zirconium (IV) chloride, kuma aka sani dazirconium tetrachloride, yana da tsarin kwayoyin ZrCl4 da nauyin kwayoyin halitta na 233.04.Yafi amfani da matsayin nazari reagents, Organic kira catalysts, waterproofing jamiái, tanning jamiái.
Sunan samfur:Zirconium chlorideZirconium tetrachloride;Zirconium (IV) chloride
Nauyin kwayoyin halitta: 233.04
EINECS: 233-058-2
Tushen tafasa: 331 (sublimation)
Yawan yawa: 2.8
Tsarin sinadarai:ZrCl4
CAS :10026-11-6
Matsakaicin narkewa:437
Ruwa mai narkewa: Mai narkewa a cikin ruwan sanyi
1. Kayayyaki
Jiki da sinadarai Properties
1. Hali: White m crystal ko foda, sauƙi deliquescent.
2. Matsayin narkewa (℃): 437 (2533.3kPa)
3. Tafasa (℃): 331 (sublimation)
4. Yawan dangi (ruwa=1): 2.80
5. Cikakken tururi matsa lamba (kPa): 0.13 (190 ℃)
6. Matsayi mai mahimmanci (MPa): 5.77
7. Solubility: Mai narkewa a cikin ruwan sanyi, ethanol, da ether, wanda ba a iya narkewa a cikin benzene, tetrachloride carbon, da carbon disulfide.
Sauƙi don ɗaukar danshi da danshi, hydrolyzed cikin hydrogen chloride da zirconium oxychloride a cikin iska mai laushi ko maganin ruwa, ma'aunin shine kamar haka:
Kwanciyar hankali
1. Kwanciyar hankali: Kwanciyar hankali
2. Abubuwan da aka haramta: ruwa, amines, alcohols, acids, esters, ketones
3. Sharuɗɗan don guje wa hulɗa: iska mai laushi
4. Polymerization haɗari: rashin polymerization
5. Samfurin lalata: chloride
2.Aikace-aikace
(1) Ana amfani da shi don yin ƙarfe zirconium, pigments, kayan kariya na yadi, abubuwan fata na fata, da sauransu.
(2) An yi amfani da shi don samar da mahadi na zirconium da kwayoyin ƙarfe na kwayoyin halitta, ana iya amfani da shi azaman kaushi da wakili mai tsarkakewa don remelted magnesium karfe, tare da sakamakon cire baƙin ƙarfe da silicon.
3.Hanyar roba
Auna zirconia da baƙar carbon da aka kayyade bisa ga ma'auni na molar, haɗa daidai da sanya su a cikin jirgin ruwan ain.Sanya kwale-kwalen ruwan a cikin bututun alin kuma sanya shi zafi zuwa 500 ℃ a cikin rafin iskar chlorine don ƙididdigewa.Tattara samfurin ta amfani da tarko a zafin jiki.Idan akai la'akari da sublimation na zirconium tetrachloride a 331 ℃, za a iya amfani da bututu mai tsayi 600mm don sake mayar da shi a cikin rafi na hydrogen gas a 300-350 ℃ don cire oxides da ferric chloride a cikin zirconium chloride.
4. Tasiri kan muhalli
Hadarin lafiya
Hanyar mamayewa: shakar numfashi, ciki, saduwa da fata.
Hatsarin Lafiya: Shakar numfashi na iya haifar da hailar numfashi, kar a hadiye.Yana da ƙarfi mai ƙarfi kuma yana iya haifar da ƙonewar fata da lalacewar ido.Gudanar da baki na iya haifar da jin zafi a cikin baki da makogwaro, tashin zuciya, amai, ɗimbin ruwa, ƙoshin jini, rugujewa, da maƙarƙashiya.
Sakamakon na yau da kullun: Yana haifar da granuloma fata.Haushi mai laushi ga sashin numfashi.
Toxicology da Muhalli
Mugun guba: LD501688mg/kg (gudanar baka ga beraye);665mg/kg (bakin linzamin kwamfuta)
Halayen haɗari: Lokacin da aka yi masa zafi ko ruwa, yakan rushe kuma ya saki zafi, yana sakin hayaki mai guba da lalata.
Konewa (bazuwar) samfur: hydrogen chloride.
Hanyar lura da dakin gwaje-gwaje: Plasma spectroscopy (Hanyar NIOSH 7300)
Ƙaddamarwa a cikin iska: tattara samfurin tare da tacewa, narkar da shi da acid, sa'an nan kuma auna shi tare da ƙwayar ƙwayar cuta ta atomatik.
Matsayin Muhalli: Safety Safety and Health Administration (1974), Matsakaicin Ma'aunin Lokacin Jirgin Sama 5.
Amsar gaggawar yabo
Ware gurɓataccen yanki, saita alamun gargaɗi kewaye da shi, da kuma ba da shawarar jami'an agajin gaggawa su sanya abin rufe fuska na iskar gas da kayan kariya na sinadarai.Kada ku yi hulɗa kai tsaye tare da kayan da aka zubar, ku guje wa ƙura, a hankali share shi, shirya wani bayani na kusan 5% ruwa ko acid, a hankali ƙara ruwan ammoniya har sai hazo ya faru, sannan a jefar da shi.Hakanan zaka iya kurkura da ruwa mai yawa, da kuma tsoma ruwan wanka a cikin tsarin ruwan sharar gida.Idan akwai babban adadin ɗigogi, cire shi a ƙarƙashin jagorancin ma'aikatan fasaha.Hanyar zubar da shara: Haɗa sharar da sodium bicarbonate, fesa da ruwan ammonia, sannan a ƙara dakakken kankara.Bayan abin ya tsaya, kurkura da ruwa a cikin magudanar ruwa.
Matakan kariya
Kariyar numfashi: Lokacin da aka fallasa ga ƙura, yakamata a sanya abin rufe fuska na gas.Saka na'urar numfashi mai ƙunshe da kai idan ya cancanta.
Kariyar ido: Saka gilashin aminci na sinadarai.
Tufafin kariya: Sanya tufafin aiki (wanda aka yi da kayan hana lalata).
Kariyar hannu: Sa safar hannu na roba.
Wani: Bayan aiki, yi wanka da canza tufafi.Ajiye tufafin da aka gurbata da guba daban kuma a sake amfani da su bayan wankewa.Kula da kyawawan halaye na tsafta.
Matakan taimakon farko
Alamar fata: Nan da nan kurkure da ruwa na akalla mintuna 15.Idan akwai kuna, nemi magani.
Tuntuɓar ido: Nan da nan ɗaga fatar ido kuma a kurkura da ruwa mai gudana ko salin ilimin lissafi na akalla mintuna 15.
Inhalation: Da sauri cire daga wurin zuwa wani wuri mai tsabta.Kula da hanyoyin numfashi mara shinge.Yi numfashi na wucin gadi idan ya cancanta.Nemi kulawar likita.
Ciwon ciki: Idan majiyyaci ya farka, sai a wanke bakinsu nan da nan, kar a yi amai, sannan a sha madara ko farin kwai.Nemi kulawar likita.
Hanyar kashe wuta: kumfa, carbon dioxide, yashi, busassun foda.
5.Hanyar ajiya
Ajiye a cikin wuri mai sanyi, bushewa, da ingantacciyar iska.Ka nisantar da tartsatsin wuta da tushen zafi.Dole ne a rufe marufi kuma a kiyaye shi daga danshi.Ya kamata a adana shi daban daga acid, amines, alcohols, esters, da dai sauransu, kuma a guji hadawa ajiya.Wurin ajiya ya kamata a sanye shi da kayan da suka dace don ɗaukar ɗigogi.
6 Na'urar Kimiya na Lissafi
1. Ƙimar magana don lissafin ma'auni na hydrophobic (XlogP): Babu
2. Yawan masu ba da gudummawar haɗin gwiwar hydrogen: 0
3. Adadin masu karɓar haɗin hydrogen: 0
4. Adadin haɗin sinadarai masu juyawa: 0
5. Yawan masu tauta: Babu
6. Topological kwayoyin polarity surface area: 0
7. Yawan atom masu nauyi: 5
8. Cajin saman: 0
9. Haɗin kai: 19.1
10. Yawan Atom na Isotope: 0
11. Ƙayyade adadin cibiyoyin tsarin atomic: 0
12. Adadin cibiyoyin gine-ginen atom: 0
13. Ƙayyade adadin cibiyoyin sitiriyo sinadarai: 0
14. Adadin abubuwan haɗin sinadarai marasa tabbas: 0
15. Yawan raka'o'in haɗin gwiwa: 1
Lokacin aikawa: Mayu-25-2023