Europium, alamar ita ce Eu, kuma lambar Atomic ita ce 63. A matsayin memba na Lanthanide, europium yawanci yana da+3 valence, amma oxygen+2 valence ma na kowa.Akwai ƙananan mahadi na europium tare da yanayin valence na +2.Idan aka kwatanta da sauran ƙananan karafa, europium ba shi da wani tasiri mai mahimmanci na ilimin halitta kuma ba shi da guba.Yawancin aikace-aikacen europium suna amfani da tasirin phosphorescence na mahaɗan Europium.Europium yana daya daga cikin mafi ƙarancin abubuwan da ke cikin sararin samaniya;Akwai kawai 5 a cikin sararin samaniya × 10-8% na abu shine europium.
Europium yana samuwa a cikin monazite
Gano Europium
Labarin ya fara ne a ƙarshen karni na 19: a wancan lokacin, ƙwararrun masana kimiyya sun fara cika sauran guraben da ke cikin tebur na lokaci-lokaci na Mendeleev ta hanyar yin nazari akan siginar Atomic.A ganina a yau, wannan aikin ba shi da wahala, kuma dalibi mai digiri na iya kammala shi;Amma a wancan lokacin, masana kimiyya kawai suna da kayan aiki masu ƙarancin inganci da samfuran da ke da wahalar tsarkakewa.Saboda haka, a cikin dukan tarihin gano Lanthanide, duk masu binciken "quasi" sun ci gaba da yin da'awar ƙarya kuma suna jayayya da juna.
A cikin 1885, Sir William Crookes ya gano sigina ta farko amma ba ta fayyace siginar kashi 63 ba: ya lura da takamaiman layin ja (609 nm) a cikin samfurin samarium.Tsakanin 1892 da 1893, wanda ya gano gallium, samarium, da dysprosium, Paul é mile LeCoq de Boisbaudran, ya tabbatar da wannan rukunin kuma ya gano wani koren band (535 nm).
Bayan haka, a cikin 1896, Eug è ne Anatole Demar ç ay haƙuri ya raba samarium oxide kuma ya tabbatar da gano wani sabon nau'in ƙasa wanda ba kasafai ba wanda ke tsakanin samarium da gadolinium.Ya yi nasarar raba wannan sinadari a cikin 1901, wanda ke nuna ƙarshen tafiya ta gano: “Ina fatan in ba wa wannan sabon rukunin Europium suna, mai alamar Eu da Atomic mass na kusan 151.”
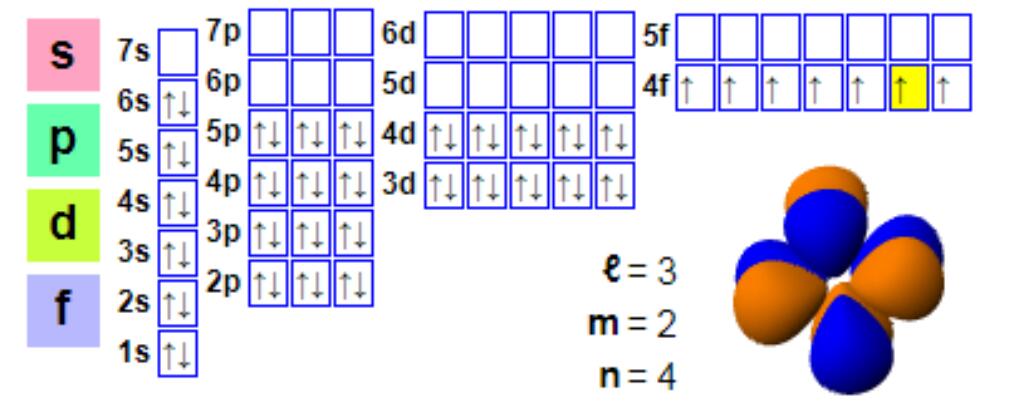
Tsarin lantarki
Tsarin lantarki:
1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p66s2 4f7
Ko da yake europium yawanci trivalent ne, yana da wuya a samar da mahadi divalent.Wannan al'amari ya bambanta da samuwar+3 valence mahadi ta mafi yawan Lanthanide.Divalent europium yana da tsarin lantarki na 4f7, yayin da rabin cika f harsashi yana samar da ƙarin kwanciyar hankali, kuma europium (II) da barium (II) suna kama da juna.Divalent europium wakili ne mai sauƙi mai ragewa wanda ke yin oxidizes a cikin iska don samar da mahadi na europium (III).A ƙarƙashin yanayin anaerobic, musamman yanayin dumama, divalent europium yana da isasshen ƙarfi kuma yana ƙoƙarin haɗa shi cikin calcium da sauran ma'adanai na ƙasa na alkaline.Wannan tsarin musayar ion shine tushen "mara kyau europium anomaly", wato, idan aka kwatanta da yawan Chondrite, yawancin ma'adanai na lanthanide irin su monazite suna da ƙananan abun ciki na europium.Idan aka kwatanta da monazite, bastnaesite sau da yawa yana nuna ƙarancin ƙarancin europium anomalies, don haka bastnaesite kuma shine babban tushen europium.
Europium karfe ne mai launin toka na baƙin ƙarfe mai narkewa na 822 ° C, wurin tafasa na 1597 ° C, da yawa na 5.2434 g/cm ³; Shi ne mafi ƙanƙanta, mafi laushi, kuma mafi ƙarancin canzawa tsakanin abubuwan da ba kasafai ba.Europium shine karfe mafi aiki a cikin abubuwan da ba kasafai ba: a dakin da zafin jiki, nan da nan ya yi hasarar ƙarfe a cikin iska kuma cikin sauri ya zama foda;Yi da ƙarfi tare da ruwan sanyi don samar da iskar hydrogen;Europium zai iya amsawa tare da boron, carbon, sulfur, phosphorus, hydrogen, nitrogen, da dai sauransu.
Aikace-aikacen Europium
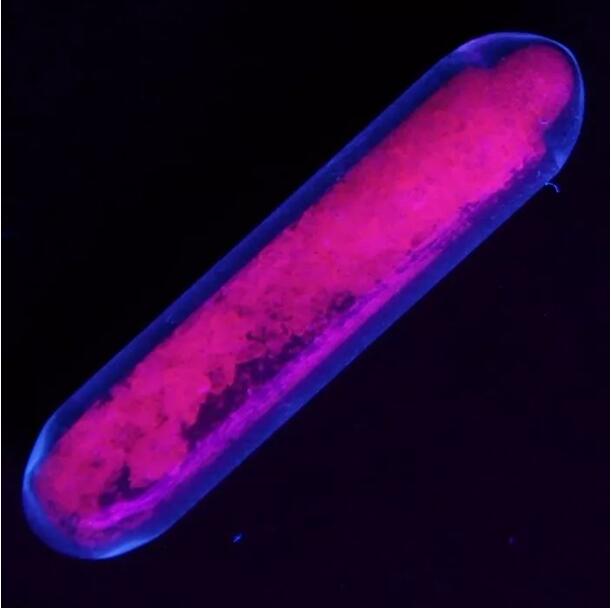
Europium sulfate yana fitar da haske mai haske a ƙarƙashin hasken ultraviolet
Georges Urbain, wani matashi ƙwararren masanin ilmin sinadarai, ya gaji kayan aikin Spectroscopy na Demar ç ay kuma ya gano cewa samfurin oxide na Yttrium (III) da aka yi tare da europium ya fitar da haske mai haske sosai a 1906. Wannan shine farkon tafiya mai nisa na kayan phosphorescent na europium - Ba wai kawai ana amfani da shi don fitar da hasken ja ba, har ma da shuɗi mai haske, saboda yanayin fitar da Eu2+ ya faɗi cikin wannan kewayon.
Fosfor wanda ya ƙunshi ja Eu3+, koren Tb3+, da blue Eu2+emitters, ko haɗin su, na iya canza hasken ultraviolet zuwa haske mai gani.Wadannan kayan suna taka muhimmiyar rawa a cikin na'urori daban-daban a duniya: na'urorin haɓaka X-ray, bututun ray na cathode ko allon plasma, da kuma fitilu masu kyalli na ceton makamashi na baya-bayan nan da diodes masu fitar da haske.
Hakanan za'a iya fahimtar tasirin tasirin yuwuwar trivalent ta hanyar kwayoyin kamshi na kwayoyin halitta, kuma ana iya amfani da irin waɗannan hadaddun a yanayi daban-daban waɗanda ke buƙatar kulawa mai zurfi, kamar tawada masu hana jabu da lambar ƙima.
Tun daga shekarun 1980s, europium yana taka rawa sosai a cikin bincike mai zurfi na biopharmaceutical ta amfani da hanyar walƙiya mai sanyi mai warware lokaci.A yawancin asibitoci da dakunan gwaje-gwaje na likita, irin wannan bincike ya zama na yau da kullun.A cikin binciken kimiyyar rayuwa, gami da hoton nazarin halittu, binciken halittu masu haske da aka yi da europium da sauran Lanthanide suna ko'ina.Abin farin ciki, kilogram daya na euro ya isa don tallafawa nazarin kusan biliyan daya - bayan da gwamnatin kasar Sin ta hana fitar da kasa da ba kasafai ba a baya-bayan nan, kasashe masu arzikin masana'antu wadanda ke firgita da karancin abubuwan adana kayan duniya ba sa damuwa da irin wannan barazana ga irin wadannan aikace-aikacen.
Ana amfani da Europium oxide azaman Ƙarfafa fitar da phosphor a cikin sabon tsarin ganewar asibiti na X-ray.Hakanan za'a iya amfani da Europium oxide don kera ruwan tabarau masu launi da masu tacewa na optoelectronic, don na'urorin ajiyar kumfa na maganadisu, da kuma cikin kayan sarrafawa, kayan kariya, da kayan tsari na injin injin atomatik.Domin atom ɗinsa na iya ɗaukar neutrons fiye da kowane sinadari, ana amfani da shi azaman abu don ɗaukar neutrons a cikin ma'aunin atomatik.
A cikin duniyar yau da take faɗaɗa cikin sauri, aikace-aikacen da aka gano kwanan nan na europium na iya yin tasiri sosai ga aikin gona.Masana kimiyya sun gano cewa robobi da aka yi da divalent europium da jan ƙarfe mara nauyi na iya juyar da ɓangaren ultraviolet na hasken rana da kyau zuwa haske mai gani.Wannan tsari yana da kore sosai (shine Madaidaicin launuka na ja).Yin amfani da irin wannan nau'in filastik don gina greenhouse zai iya ba da damar tsire-tsire su sami haske mai haske da kuma ƙara yawan amfanin gona da kusan 10%.
Hakanan za'a iya amfani da Europium zuwa kwakwalwan ƙwaƙwalwar ajiyar ƙididdiga, waɗanda za su iya dogara da adana bayanai na kwanaki da yawa a lokaci guda.Waɗannan za su iya ba da damar adana bayanan ƙididdiga masu mahimmanci a cikin na'ura mai kama da rumbun kwamfyuta da jigilar kaya a cikin ƙasar.
Lokacin aikawa: Juni-27-2023