Menenekasa kasa?
Dan Adam yana da tarihin sama da shekaru 200 tun bayan gano kasa da ba kasafai ba a shekarar 1794. Tun da akwai karancin ma'adinan da ba kasafai aka samu ba a wancan lokacin, kadan ne kawai na oxides da ba za a iya narkewa ba ta hanyar sinadarai. A tarihi, ana kiran irin waɗannan oxides “ƙasa”, don haka sunan ƙasa mai wuya.
A gaskiya ma, ma'adinan da ba kasafai ba ne a yanayi. Rare ƙasa ba ƙasa ba ne, amma nau'in ƙarfe ne na yau da kullun. Its aiki type ne kawai na biyu zuwa alkali karafa da alkaline duniya karafa. Suna da ƙarin abun ciki a cikin ɓawon burodi fiye da jan ƙarfe na kowa, zinc, tin, cobalt, da nickel.
A halin yanzu, kasa da ba kasafai ake amfani da su ba a fannoni daban-daban kamar na’urorin lantarki, sinadaran petrochemicals, karafa da sauransu, kusan duk bayan shekaru 3-5, masana kimiyya kan gano sabbin abubuwan amfani da kasa da ba kasafai suke amfani da su ba, kuma a cikin duk wasu abubuwa shida da aka kirkira, mutum ba zai iya yin hakan ba sai da kasa mai wuya.
Kasar Sin tana da wadatar ma'adinan kasa da ba kasafai ba, tana matsayi na daya a matsayi na uku a duniya: ma'auni, ma'aunin samarwa, da yawan fitarwa zuwa kasashen waje. A sa'i daya kuma, kasar Sin ita ce kasa daya tilo da za ta iya samar da dukkan karafa 17 da ba kasafai ba, musamman ma matsakaita da nauyi da ba kasafai ake amfani da su ba tare da fitattun kayan aikin soja.
Rare ƙasa kashi abun da ke ciki
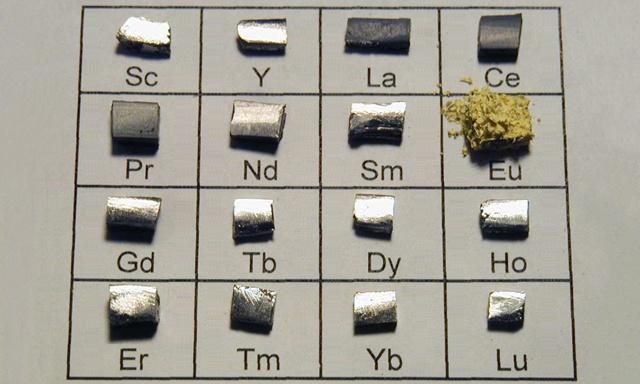
Abubuwan da ba kasafai suke yin kasa ba sun hada da abubuwan Lanthanide a cikin tebirin abubuwan sinadaran lokaci-lokaci:lantanum(La)cerium(Ce),praseodymium(Pr),neodymium(Nd), promethium (Pm),samari(Sm),europium(Eu),gadolinium(Gd),terbium(Tb),dysprosium(Dy),holmium(Ho),erbium(Eh),thulium(Tm),ytterbium(Yb),Lutium(Lu), da abubuwa biyu masu alaƙa da lanthanide:scandium(Sc) kumayttrium(Y).

Ana kirantaRare Duniya, wanda aka takaita da Rare Duniya.
Rarraba abubuwan da ba kasafai ba a duniya
An rarraba su ta hanyar zahiri da sinadarai na abubuwa:
Abubuwan da ba kasafai ba a kasa:Scandium, yttrium, lanthanum, cerium, praseodymium, neodymium, promethium, samarium, europium
Abubuwa na ƙasa marasa nauyi:Ya ƙunshi terbium, gadolinium, terbium, dysprosium, holmium, erbium, thulium, ytterbium, lutetium.
Rarraba ta halayen ma'adinai:
Ƙungiyar Cerium:Lanthanum, cerium, praseodymium, neodymium, promethium, samarium, europium
Ƙungiyar Yttrium:Daga cikin su akwai terbium, gadolinium, terbium, dysprosium, holmium, erbium, thulium, ytterbium, lutetium, scandium, yttrium.
Rabewa ta hanyar rabuwa:
Ƙasa mai ƙarancin haske (P204 raunin acidity hakar)Lanthanum, cerium, praseodymium, neodymium
Ƙasar da ba kasafai ba (P204 low acidity hakar):samarium, europium, gadolinium, terbium, dysprosium
Kasa mai nauyi mai nauyi (hakar acidity a cikin P204):thulium, erbium, thulium, ytterbium, lutium, yttrium.
Abubuwan abubuwan da ba kasafai ba a duniya
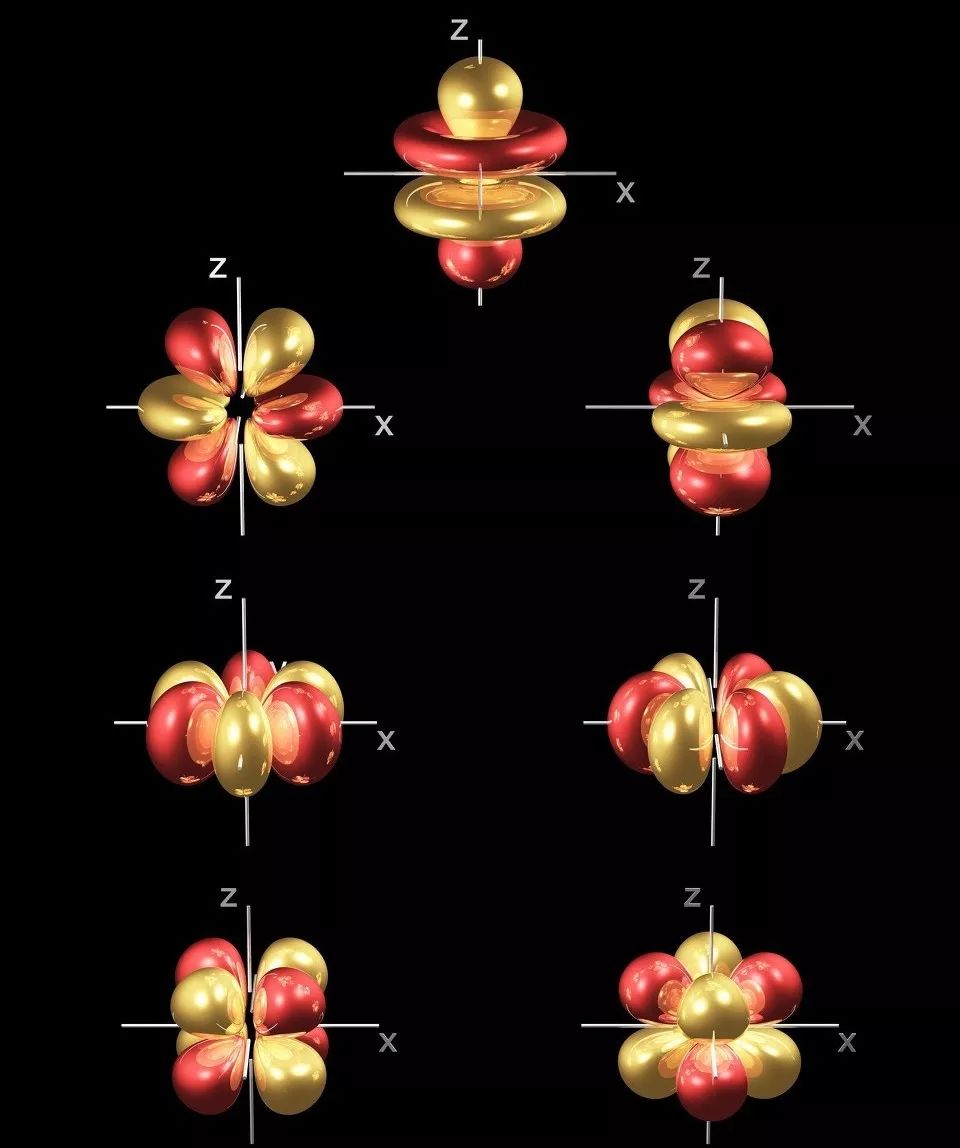
Fiye da ayyuka 50 na abubuwan da ba kasafai ba na duniya suna da alaƙa da tsarin lantarki na musamman na 4f, wanda ke sa su yi amfani da su sosai a cikin kayan gargajiya da sabbin kayan fasaha na zamani.
1. Halin jiki da sinadarai
★ Yana da sifofi na karafa; Yana da launin toka na azurfa, banda praseodymium da neodymium, yana bayyana rawaya mai haske
★ Wadancan kalar oxide
★ Samar da barga mahadi tare da wadanda ba karafa
★ Karfe mai rai
★ Sauƙin oxidize a cikin iska
2 Optoelectronic Properties
★ Sublayer 4f wanda ba a cika ba, inda 4f electrons ke da kariya ta waje na electrons, wanda ke haifar da kalmomi daban-daban da matakan makamashi.
Lokacin da 4f electrons canzawa, za su iya sha ko fitar da radiation na daban-daban raƙuman ruwa daga ultraviolet, bayyane zuwa infrared yankuna, sa su dace da matsayin luminescent kayan.
★ Good conductivity, iya shirya rare duniya karafa ta electrolysis Hanyar
Matsayin 4f Electrons na Rare Abubuwan Abubuwan Duniya a Sabbin Kayayyaki
1.Materials masu amfani da kayan lantarki na 4f
★ 4f tsarin jujjuyawar lantarki:bayyana a matsayin mai ƙarfi magnetism - dace don amfani azaman kayan maganadisu na dindindin, kayan hoton MRI, firikwensin magnetic, superconductor, da sauransu.
★ 4f orbital electron transfer: bayyana a matsayin luminescent Properties - dace da amfani a matsayin luminescent kayan kamar phosphors, infrared Laser, fiber amplifiers, da dai sauransu
Canje-canje na lantarki a cikin ƙungiyar jagorar matakin makamashi na 4f: bayyana azaman kayan canza launi - dace da canza launi da kuma lalata abubuwan abubuwan tabo mai zafi, pigments, mai yumbu, gilashi, da sauransu.
2 yana da alaƙa kai tsaye da 4f electron, ta amfani da radius Ionic, caji da kaddarorin sinadarai
★ Sifofin nukiliya:
Small thermal neutron Absorption cross sashe - dace don amfani a matsayin tsarin kayan aikin nukiliya reactors, da dai sauransu
Babban sashin shayarwar neutron - wanda ya dace da kayan kariya na injin nukiliya, da sauransu
★ Rare ƙasa Ionic radius, caji, jiki da sinadarai Properties:
Lattice lahani, irin wannan radius Ionic, sinadarai Properties, daban-daban zargin - dace da dumama, mai kara kuzari, ji kashi, da dai sauransu
Ƙayyadaddun tsari - dace don amfani azaman kayan haɗin gwanon cathode na ajiya na hydrogen, kayan sha na microwave, da dai sauransu
Electro Tantancewar da dielectric Properties - dace don amfani da haske modulation kayan, m tukwane, da dai sauransu
Lokacin aikawa: Yuli-06-2023