Scandium, tare da alamar element Sc da lambar Atomic na 21, yana da sauƙin narkewa a cikin ruwa, yana iya hulɗa da ruwan zafi, kuma cikin sauƙi yana yin duhu a cikin iska. Babban darajarsa shine +3. Yawancin lokaci ana haɗe shi da gadolinium, erbium, da sauran abubuwa, tare da ƙananan yawan amfanin ƙasa da abun ciki na kusan 0.0005% a cikin ɓawon burodi. Ana amfani da Scandium sau da yawa don yin gilashin musamman da gauraye masu zafi masu nauyi.
A halin yanzu, da tabbatar reserves na scandium a duniya ne kawai 2 ton miliyan, 90 ~ 95% na wanda aka kunshe a cikin Bauxite, phosphorite da baƙin ƙarfe titanium ores, da kuma wani karamin sashi a cikin uranium, thorium, tungsten da rare duniya ores, yafi rarraba a Rasha, China, Tajikistan, Madagascar, Norway da sauran ƙasashe. Kasar Sin tana da wadatar albarkatun scandium, tare da dimbin ma'adinan da ke da alaka da scandium. Bisa kididdigar da ba ta cika ba, yawan ajiyar na'urar scandium a kasar Sin ya kai ton 600000, wadanda ke kunshe a cikin Bauxite da phosphorite adibas, porphyry da quartz vein tungsten adibas a kudancin kasar Sin, da kasa kasa kasa ajiya a kudancin kasar Sin, Bayan Obo rare earth iron ore ajiya a ciki Mongolia, da Panchunium magnet vanaite ajiya na Sinadium.
Sakamakon karancin sinadari, shi ma farashin scandium ya yi tsada sosai, kuma a lokacin da ya kai kololuwar sa, an kara tsadar sikandimu zuwa farashin zinare sau 10. Duk da cewa farashin scandium ya fadi, amma har yanzu ya ninka farashin zinare sau hudu!
Gano Tarihi
A cikin 1869, Mendeleev ya lura da gibi a cikin adadin atomic tsakanin calcium (40) da titanium (48), kuma ya yi annabta cewa akwai matsakaicin matsakaicin sinadarin atomic a nan. Ya annabta cewa oxide ɗinsa shine X ₂ O Å. An gano Scandium a cikin 1879 ta Lars Frederik Nilson na Jami'ar Uppsala a Sweden. Ya ciro ta ne daga ma'adinan zinare na baki, wani hadadden tama mai dauke da nau'in karfe 8. Ya ciroErbium (III) oxidedaga baƙar fata m zinariya tama, kuma samuYtterbium (III) oxidedaga wannan oxide, kuma akwai wani oxide mai sauƙi, wanda bakansa ya nuna cewa ƙarfe ne wanda ba a san shi ba. Wannan shi ne karfen da Mendeleev ya annabta, wanda oxide neSc₂O₃. Karfe na scandium da kansa ya fito dagaScandium chlorideta hanyar narkewar electrolytic a cikin 1937.
Mendeleev
Tsarin lantarki
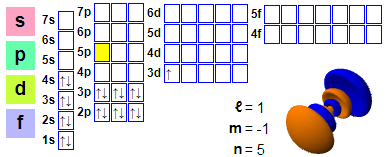
Tsarin lantarki: 1s2 2s2 2p6 3s2 3p6 4s2 3d1
Scandium karfe ne mai laushi, fari na azurfa tare da wurin narkewa na 1541 ℃ da wurin tafasa na 2831 ℃.
Tsawon lokaci mai tsawo bayan gano shi, ba a nuna amfani da scandium ba saboda wahalar samarwa. Tare da haɓaka hanyoyin rarrabuwar abubuwan da ba kasafai ba a duniya, yanzu akwai babban tsari mai gudana don tsarkake mahaɗan scandium. Saboda scandium kasa da alkaline fiye da yttrium da Lanthanide, hydroxide shine mafi rauni, don haka za'a raba nau'ikan ma'adinan ƙasa da ba kasafai ba wanda ke ɗauke da sinadari mai rahusa da ƙarancin ƙasa ta hanyar "hazo mataki" lokacin da Scandium (III) hydroxide ke bi da ammonia bayan an canza shi zuwa mafita. Wata hanyar ita ce raba Scandium nitrate ta hanyar bazuwar nitrate na Polar. Saboda scandium nitrate shine mafi sauƙi don bazuwa, ana iya raba scandium. Bugu da ƙari, cikakkiyar farfadowa na rakiyar scandium daga uranium, thorium, tungsten, tin da sauran ma'adinan ma'adinai shima muhimmin tushen scandium ne.
Bayan samun madaidaicin fili na scandium, ana juye shi zuwa ScCl Å kuma an narke tare da KCl da LiCl. Ana amfani da narkakkar zinc a matsayin cathode don electrolysis, yana haifar da scandium don hazo akan lantarki na zinc. Sa'an nan, zinc yana ƙafe don samun ƙwayar ƙarfe. Wannan farin ƙarfe ne mai nauyi mai nauyi tare da sinadarai masu aiki sosai, wanda zai iya amsawa da ruwan zafi don samar da iskar hydrogen. Don haka wannan karfen scandium da kuke gani a wannan hoton an kulle shi a cikin kwalba sannan kuma ana kiyaye shi da iskar argon, idan ba haka ba, scandium zai yi sauri ya samar da wani duhu mai duhu mai launin rawaya ko launin toka, ya rasa hasken karfe mai sheki.
Aikace-aikace
Masana'antar hasken wuta
Amfani da scandium ya ta'allaka ne a wurare masu haske sosai, kuma ba ƙari ba ne a kira shi Ɗan Haske. Makamin sihiri na farko na scandium shine ake kira scandium sodium lamp, wanda za'a iya amfani dashi don kawo haske ga dubban gidaje. Wannan ƙarfe halide Hasken lantarki: kwan fitila yana cike da Sodium iodide da Scandium triiodide, kuma ana ƙara scandium da foil sodium a lokaci guda. A lokacin fitar da wutar lantarki mai ƙarfi, ions scandium da ions sodium bi da bi suna fitar da hasken siffar ficewar su. Layukan sikelin na sodium sune 589.0 da 589.6 nm, shahararrun fitilun rawaya guda biyu, yayin da layin sikelin na scandium shine 361.3 ~ 424.7 nm, jerin kusa da ultraviolet da shuɗi mai haske. Domin sun dace da juna, gaba ɗaya launin hasken da aka samar shine farin haske. Shi ne daidai saboda scandium sodium fitilu suna da halaye na high haske yadda ya dace, mai kyau haske launi, ikon ceton, dogon sabis rayuwa, da kuma karfi hazo karya ikon cewa za a iya amfani da ko'ina ga talabijin kyamarori, murabba'ai, wasanni wuraren, da kuma hanya lighting, kuma an san su a matsayin ƙarni na uku haske kafofin. A kasar Sin, ana ci gaba da bunkasa irin wannan nau'in fitila sannu a hankali a matsayin sabuwar fasaha, yayin da a wasu kasashen da suka ci gaba, an fara amfani da irin wannan fitila tun farkon shekarun 1980.
Makamin sihiri na biyu na scandium shine sel photovoltaic na hasken rana, wanda zai iya tattara hasken da ya warwatse a ƙasa ya mai da shi wutar lantarki don korar al'ummar ɗan adam. Scandium shine mafi kyawun ƙarfe mai shinge a cikin ƙarfe mai insulator semiconductor silicon hasken rana da ƙwayoyin rana.
Makamin sihirinsa na uku ana kiransa γ A ray source, wannan makamin sihirin yana iya haskakawa da kansa, amma irin wannan haske ba zai iya samun ido a ido ba, shi ne kwararar photon mai ƙarfi. Yawancin lokaci muna fitar da 45Sc daga ma'adanai, wanda shine kawai isotopes na halitta na scandium. Kowane tsakiya na 45Sc ya ƙunshi protons 21 da neutrons 24. 46Sc, isotope na wucin gadi na rediyoaktif, ana iya amfani da shi azaman γ Maɓuɓɓugan Radiation ko kuma za a iya amfani da atom ɗin ganowa don aikin rediyo na muggan ciwace-ciwace. Akwai kuma aikace-aikace kamar yttrium gallium scandium garnet laser,Scandium fluoridegilashin infrared Optical fiber, da scandium mai rufi cathode ray tube akan talabijin. Da alama an haifi scandium tare da haske.
Alloy masana'antu
An yi amfani da Scandium a farkon nau'insa don amfani da alluran alluran doping. Muddin an ƙara 'yan dubbai na scandium zuwa aluminum, za a samar da wani sabon lokaci na Al3Sc, wanda zai taka rawar Metamorphism a cikin aluminum gami kuma ya sa tsari da kaddarorin haɗin gwal ya canza sosai. Ƙara 0.2% ~ 0.4% Sc (wanda yake da kama da rabon ƙara gishiri don motsa kayan lambu mai soyayyen a gida, kawai ana buƙata kaɗan) zai iya ƙara yawan zafin jiki na recrystallization na gami da 150-200 ℃, kuma yana inganta ƙarfin zafi mai girma, kwanciyar hankali tsarin, aikin walda, da juriya na lalata. Hakanan zai iya guje wa al'amuran haɓakawa wanda ke da sauƙin faruwa yayin aiki na dogon lokaci a yanayin zafi. High ƙarfi da high taurin aluminum gami, sabon high-ƙarfi lalata-resistant weldable aluminum gami, sabon high-zazzabi aluminum gami, high-ƙarfi neutron sakawa a iska mai guba aluminum gami, da dai sauransu, da sosai m ci gaban al'amurra a cikin jirgin sama, jirgin sama, jiragen ruwa, nukiliya reactors, haske motocin da high-gudun jiragen kasa.
Scandium kuma babban mai gyara ƙarfe ne, kuma ƙaramin adadin scandium na iya inganta ƙarfi da taurin ƙarfen simintin. Bugu da ƙari, ana iya amfani da scandium a matsayin ƙari don tungsten mai zafin jiki da chromium gami. Tabbas, baya ga yin tufafin aure ga wasu, scandium yana da mahimmin narkewa kuma yawansa yana kama da aluminum, kuma ana amfani da shi a babban narkewar ma'aunin nauyi kamar su scandium titanium alloy da scandium magnesium alloy. Koyaya, saboda tsadar sa, ana amfani da shi gabaɗaya a manyan masana'antun masana'antu kamar su jiragen sama da rokoki.
Kayan yumbura
Scandium, abu guda ɗaya, ana amfani dashi gabaɗaya a cikin allurai, kuma oxides ɗinsa suna taka muhimmiyar rawa a cikin kayan yumbu kamar haka. Tetragonal zirconia yumbu abu, wanda za'a iya amfani da shi azaman kayan lantarki don ƙwararrun man fetur mai oxide, yana da dukiya ta musamman inda aikin wannan electrolyte ya karu tare da karuwar zafin jiki da kuma iskar oxygen a cikin yanayi. Duk da haka, tsarin crystal na wannan kayan yumbu da kansa ba zai iya wanzuwa a tsaye kuma ba shi da darajar masana'antu; Wajibi ne a yi amfani da wasu abubuwa da za su iya gyara wannan tsarin don kiyaye ainihin abubuwan da suka dace. Ƙara 6 ~ 10% Scandium oxide kamar tsari ne na kankare, don haka za a iya daidaita zirconia akan lattice mai murabba'i.
Hakanan akwai kayan aikin yumbu na injiniya kamar ƙarfi mai ƙarfi da ƙarfin zafin jiki na silicon nitride azaman densifiers da stabilizers.
A matsayin densifier,Scandium oxidezai iya samar da wani lokaci mai jujjuyawa Sc2Si2O7 a gefen kyawawan barbashi, don haka rage girman nakasar yumbu injiniyoyi. Idan aka kwatanta da sauran oxides, zai iya inganta ingantaccen kayan aikin injiniya mai zafi na silicon nitride.
Catalytic kimiyya
A cikin injiniyan sinadarai, ana amfani da scandium sau da yawa a matsayin mai haɓakawa, yayin da Sc2O3 za a iya amfani dashi don rashin ruwa da deoxidation na ethanol ko isopropanol, lalata acetic acid, da samar da ethylene daga CO da H2. Pt Al mai kara kuzari wanda ke dauke da Sc2O3 shima muhimmin mai kara kuzari ne don tsarkakewar hydrogenation mai nauyi da tafiyar matakai na tacewa a cikin masana'antar petrochemical. A cikin halayen fashewar catalytic kamar Cumene, aikin Sc-Y zeolite mai haɓakawa ya ninka sau 1000 sama da na Aluminum silicate catalyst; Idan aka kwatanta da wasu masu kara kuzari na al'ada, haɓakar haɓakar abubuwan haɓakawa na scandium za su yi haske sosai.
Masana'antar makamashin nukiliya
Ƙara ƙaramin adadin Sc2O3 zuwa UO2 a cikin makamashin nukiliya mai zafi mai zafi zai iya guje wa canjin lattice, haɓaka girma, da fashewar da UO2 zuwa U3O8 ya haifar.
Kwayoyin mai
Hakazalika, ƙara 2.5% zuwa 25% scandium zuwa batirin nickel alkali zai ƙara rayuwar sabis ɗin su.
Noma kiwo
A cikin aikin noma, tsaba irin su masara, gwoza, fis, alkama da sunflower za a iya bi da su tare da Scandium sulfate (yawan taro shine gabaɗaya 10-3 ~ 10-8mol / L, tsire-tsire daban-daban za su sami daban-daban), kuma an sami sakamako na ainihi na haɓaka germination. Bayan sa'o'i 8, bushewar nauyin tushen da buds ya karu da 37% da 78% bi da bi idan aka kwatanta da seedlings, amma har yanzu ana kan binciken tsarin.
Daga hankalin Nielsen ga bashin bayanan Atomic mass data zuwa yau, scandium ya shiga hangen nesa na mutane kawai shekaru dari ko ashirin, amma ya kusan zama a kan benci na tsawon shekaru ɗari. Sai da ƙwaƙƙwaran ci gaban kimiyyar abin duniya a ƙarshen karnin da ya gabata ya kawo kuzari gare shi. A yau, abubuwan da ba kasafai ba a duniya, ciki har da scandium, sun zama taurari masu zafi a kimiyyar kayan aiki, suna taka rawar da ke canzawa koyaushe a cikin dubban tsarin, suna kawo mafi dacewa ga rayuwarmu a kowace rana, da ƙirƙirar ƙimar tattalin arziƙi wanda ma ya fi wuya a auna.
Lokacin aikawa: Juni-29-2023