Lutetiumwani nau'in ƙasa ne da ba kasafai ba tare da tsada mai tsada, ƙaramin tanadi, da iyakacin amfani. Yana da taushi kuma mai narkewa a cikin acid ɗin dilute, kuma yana iya amsawa da ruwa sannu a hankali.
Isotopes da ke faruwa a zahiri sun haɗa da 175Lu da rabin rayuwa na 2.1 × 10 ^ 10 mai shekaru β Emitter 176Lu. Ana yin ta ta hanyar rage Lutetium (III) fluoride LuF ∨ · 2H ₂ O tare da calcium.
Babban amfani shine a matsayin mai kara kuzari ga fashewar man fetur, alkylation, hydrogenation, da halayen polymerization; Bugu da ƙari, Lutetium tantalate kuma za a iya amfani dashi azaman kayan aikin X-ray fluorescent foda; 177Lu, radionuclide, ana iya amfani da shi don maganin rediyo na ciwace-ciwace.

Gano Tarihi
An gano shi: G. Urban
An gano shi a cikin 1907
An raba Lutetium daga ytterbium ta hannun masanin kimiyar Faransa Ulban a cikin 1907 kuma shi ma wani abu ne da ba kasafai aka gano shi ba kuma aka tabbatar dashi a farkon karni na 20. Sunan Latin na Lutium ya fito ne daga tsohuwar sunan Paris, Faransa, wanda shine wurin haifuwar Urban. Gano Lutium da wani nau'in ƙasa mai ƙarancin gaske na europium ya kammala gano dukkan abubuwan da ba kasafai suke cikin ƙasa ba. Za a iya la'akari da gano su a matsayin buɗe kofa na huɗu don gano abubuwan da ba kasafai ake samun su ba da kuma kammala mataki na huɗu na gano abubuwan da ba kasafai ba.
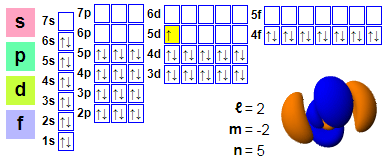
Tsarin lantarki
Shirye-shiryen lantarki:
1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d1
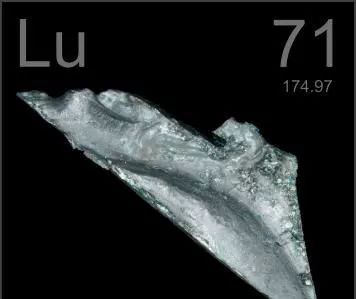
Lutetium karfe ne fari na azurfa, wanda shine karfe mafi wuya kuma mafi girma a tsakanin abubuwan da ba kasafai ba a duniya; Matsayin narkewa 1663 ℃, wurin tafasa 3395 ℃, yawa 9.8404. Lutetium yana da kwanciyar hankali a cikin iska; Lutetium oxide shine crystal mara launi wanda ke narkewa cikin acid don samar da gishiri mara launi daidai.
Ƙarfe mai ƙyalli na luteium yana tsakanin azurfa da baƙin ƙarfe. Abubuwan da ke cikin ƙazanta suna da tasiri mai mahimmanci a kan kaddarorin su, don haka sau da yawa ana samun bambance-bambance masu mahimmanci a cikin kayan jikinsu a cikin wallafe-wallafe.
Metal yttrium, gadolinium, da lutetium suna da juriya mai ƙarfi kuma suna iya kula da ƙarancin ƙarfe na dogon lokaci.
Aikace-aikace
Saboda matsalolin samarwa da farashi mai yawa, luteium yana da ƴan amfanin kasuwanci. Abubuwan da ke cikin luteium ba su bambanta da sauran karafa na lanthanide ba, amma ajiyarsa ba su da yawa, don haka a wurare da yawa, ana amfani da sauran karafa na lanthanide don maye gurbin lutetium.
Ana iya amfani da Lutetium don yin wasu nau'i na musamman, irin su Lutetium aluminum gami za a iya amfani da su don nazarin kunnawa Neutron. Hakanan za'a iya amfani da Lutetium azaman mai kara kuzari don fashewar man fetur, alkylation, hydrogenation, da halayen polymerization. Bugu da ƙari, doping lutetium a cikin wasu lu'ulu'u na Laser kamar Yttrium aluminum garnet na iya inganta aikin laser da daidaiton gani. Bugu da ƙari, ana iya amfani da lutetium don phosphor: Lutetium tantalate shine mafi ƙarancin farin abu da aka sani a halin yanzu, kuma abu ne mai kyau don X-ray phosphor.
177Lu shine radionuclide na roba, wanda za'a iya amfani dashi don radiotherapy na ciwace-ciwacen daji.
Lutetium oxidedoped cerium yttrium lutium silicate crystal
Lokacin aikawa: Juni-26-2023