Daga cikin wadanda ba siliceous oxides, alumina yana da kyau inji Properties, high zafin jiki juriya da kuma lalata juriya, yayin da mesoporous alumina (MA) yana daidaitacce pore size, babban takamaiman surface yankin, babban pore girma da kuma low samar da kudin, wanda aka yadu amfani a catalysis, sarrafawa miyagun ƙwayoyi saki, adsorption da sauran filayen, irin su fatattaka, hydrocracking da hydrodesulfurrous masana'antu alumina fiye da amfani da petrole raw kayan alumina. kai tsaye zai shafi aikin alumina, rayuwar sabis da zaɓi na mai kara kuzari. Misali, a cikin aikin tsarkake shaye-shayen ababen hawa, gurbacewar da aka samu daga sinadaran man injuna za su samar da coke, wanda hakan zai haifar da toshewar pores mai kara kuzari, wanda hakan zai rage ayyukan kara kuzari. Za'a iya amfani da surfactant don daidaita tsarin jigilar alumina don samar da MA.Inganta aikin kuzarinsa.
MA yana da takurawa, kuma ana kashe karafa masu aiki bayan ƙididdige yawan zafin jiki. Bugu da ƙari, bayan ƙididdige yawan zafin jiki, tsarin mesoporous ya rushe, MA kwarangwal yana cikin yanayin amorphous, kuma acidity na saman ba zai iya cika bukatunsa a fagen aiki ba. Ana buƙatar gyaran gyare-gyare sau da yawa don inganta aikin catalytic, tsarin tsarin mesoporous, kwanciyar hankali na thermal da kuma acidity na kayan MA. Ƙungiyoyin gyare-gyare na yau da kullum sun haɗa da heteroatoms na karfe (Fe, Co, Ni, Cu, Zn, Pd, Pt, Zr, da dai sauransu) da kuma karfe oxides (TiO2, NiO, Co3O4, REO2, Cuad, da dai sauransu). cikin kwarangwal.
Tsarin lantarki na musamman na abubuwan duniya da ba kasafai ba ya sanya mahadin sa suna da kaddarorin gani na musamman, na lantarki da na maganadisu, kuma ana amfani da su a cikin kayan haɓaka, kayan lantarki, kayan talla da kayan maganadisu. Rare ƙasa modified mesoporous kayan iya daidaita acid (alkali) dukiya, ƙara oxygen sarari, da kuma hada karfe nanocrystalline kara kuzari da uniform watsawa da barga nanometer scale.Appropriate porous kayan da rare ƙasa iya inganta surface watsawa na karfe nanocrystals da kwanciyar hankali da kuma carbon jijiya juriya na catalysts. A cikin wannan takarda, za a gabatar da gyare-gyaren ƙasa da ba kasafai ba da aikin MA don haɓaka aikin haɓaka, kwanciyar hankali na thermal, ƙarfin ajiyar iskar oxygen, takamaiman yanki da tsarin pore.
1 MA shiri
1.1 shirye-shiryen mai ɗaukar alumina
Hanyar shirye-shiryen mai ɗaukar alumina tana ƙayyade rarraba tsarin pore, kuma hanyoyin shirye-shiryenta na yau da kullun sun haɗa da hanyar rashin ruwa na pseudo-boehmite (PB) da hanyar sol-gel. Pseudoboehmite (PB) Calvet ne ya fara gabatar da shi, kuma H+ ya haɓaka peptization don samun γ-AlOOH colloidal PB mai ɗauke da ruwan interlayer, wanda aka ƙididdige shi kuma ya bushe a babban zafin jiki don samar da alumina. A cewar daban-daban albarkatun kasa, shi ne sau da yawa raba zuwa hazo hanya, carbonization hanya da kuma alcoholaluminum hydrolysis method.The colloidal solubility na PB ya shafi crystallinity, kuma an inganta shi tare da karuwa na crystallinity, da kuma an shafi aiki sigogi sigogi.
Yawancin lokaci ana shirya PB ta hanyar hazo. Ana zuba Alkaki a cikin ruwan aluminate ko kuma a zuba acid a cikin ruwan aluminate a zuba a samu ruwa mai ruwa (alkali hazo), ko kuma a samu ruwan aluminate a samu alumina monohydrate, sai a wanke, a bushe sannan a datse a samu PB. Hanyar hazo yana da sauƙi don aiki da ƙananan farashi, wanda aka yi amfani da shi sau da yawa a cikin samar da masana'antu, amma yana rinjayar abubuwa da yawa (maganin pH, maida hankali, zafin jiki, da dai sauransu) .Kuma wannan yanayin don samun ƙwayar cuta tare da mafi kyawun rarrabawa yana da tsanani. A cikin hanyar carbonization, Al (OH) 3 yana samuwa ta hanyar amsawar CO2 da NaAlO2, kuma ana iya samun PB bayan tsufa. Wannan hanya tana da abũbuwan amfãni daga aiki mai sauƙi, high quality samfurin, babu gurbatawa da kuma low cost, kuma zai iya shirya alumina tare da high catalytic aiki, m lalata juriya da kuma high takamaiman surface yankin tare da low zuba jari da kuma high koma.Aluminum alkoxide hydrolysis Hanyar da ake amfani da sau da yawa don shirya high-tsarki PB. Aluminum alkoxide ne hydrolyzed don samar da aluminum oxide monohydrate, sa'an nan kuma bi da samun high-tsarki PB, wanda yana da kyau crystallinity, uniform barbashi size, mayar da hankali pore size rarraba da kuma high mutunci na mai siffar zobe barbashi. Duk da haka, tsarin yana da wuyar gaske, kuma yana da wuya a warkewa saboda amfani da wasu abubuwan da ake amfani da su masu guba.
Bugu da kari, ana amfani da gishirin inorganic ko sinadarai na karafa don shirya abubuwan da ake so alumina ta hanyar sol-gel, sannan ana saka ruwa mai tsafta ko abubuwan kaushi don shirya mafita don samar da sol, sai a yi gelli, a bushe sannan a gasa. A halin yanzu, tsarin shirye-shiryen alumina har yanzu yana inganta akan hanyar PB dehydration, kuma hanyar carbonization ta zama babbar hanyar samar da alumina masana'antu saboda tattalin arzikinta da kare muhalli.
1.2 MA shiri
Alumina na al'ada ba zai iya biyan buƙatun aikin ba, don haka ya zama dole don shirya babban aiki MA. Hanyoyin kira yawanci sun haɗa da: Hanyar simintin nano tare da ƙirar carbon azaman samfuri mai wuya; Haɗin kai na SDA: Tsarin Haɗuwa da Haɓakawa (EISA) a gaban samfura masu laushi kamar SDA da sauran cationic, anionic ko nonionic surfactants.
1.2.1 Tsarin EISA
Ana amfani da samfuri mai laushi a cikin yanayin acidic, wanda ke guje wa tsarin rikitarwa da cin lokaci na hanyar membrane mai wuya kuma zai iya gane ci gaba da gyaran fuska na budewa. Shirye-shiryen MA ta EISA ya ja hankalin mutane da yawa saboda sauƙin samuwa da sake haifuwa. Za a iya shirya tsarin mesoporous daban-daban. The pore size of MA za a iya gyara ta hanyar canza hydrophobic sarkar tsawon surfactant ko daidaita molar rabo na hydrolysis kara kuzari zuwa aluminum precursor a cikin solution.Saboda haka, EISA, kuma aka sani da daya mataki kira da gyare-gyare sol-gel Hanyar high surface area MA da kuma oda mesoporous alumina (OMA), An yi amfani da daban-daban taushi shaci, trithan123 shaci, kamar 1 F. da dai sauransu EISA iya maye gurbin da co-taro tsari na organoaluminum precursors, kamar aluminum alkoxides da surfactant shaci, yawanci aluminum isopropoxide da P123, domin samar da mesoporous kayan.The nasara ci gaban EISA tsari na bukatar daidai daidaitawa na hydrolysis da condensation kinetics don samun barga soltant kafa a cikin surfactant solfact.
A cikin tsarin EISA, yin amfani da abubuwan da ba na ruwa ba (irin su ethanol) da ma'aikatan hadaddun kwayoyin halitta na iya rage jinkirin haɓakar hydrolysis da adadin kuzari na precursors na organoaluminum da kuma haifar da haɗin kai na kayan OMA, kamar Al (OR) 3 da aluminum isopropoxide. Koyaya, a cikin masu kaushi maras ruwa, samfuran surfactant yawanci suna rasa hydrophilicity/hydrophobicity. Bugu da ƙari, Saboda jinkiri na hydrolysis da polycondensation, matsakaicin samfurin yana da ƙungiyar hydrophobic, wanda ya sa ya zama da wuya a yi hulɗa tare da samfurin surfactant. Sai kawai lokacin da maida hankali na surfactant da digiri na hydrolysis da polycondensation na aluminum suna sannu a hankali a kan aiwatar da ƙaura evaporation iya zama kai taro na samfuri da aluminum. Saboda haka, da yawa sigogi da suka shafi evaporation yanayi na kaushi da kuma hydrolysis da condensation dauki precursors, irin su zazzabi, dangi zafi, kara kuzari, sauran ƙarfi evaporation kudi, da dai sauransu, zai shafi karshe taro tsarin. Kamar yadda aka nuna a cikin fig. 1, OMA kayan tare da high thermal kwanciyar hankali da kuma high catalytic aiki aka hada ta solvothermal taimaka evaporation jawo kai taro (SA-EISA). solvothermal magani inganta cikakken hydrolysis na aluminum precursors don samar da kananan-sized gungu aluminum hydroxyl kungiyoyin, wanda inganta hulda tsakanin surfactants da aluminum.Biyu-girma hexagonal mesophase aka kafa a cikin EISA tsari da calcined a 400 ℃ don samar da OMA abu. A cikin tsarin EISA na gargajiya, tsarin zubar da ruwa yana tare da hydrolysis na organoaluminum precursor, don haka yanayin ƙaura yana da tasiri mai mahimmanci akan amsawa da tsarin ƙarshe na OMA. Matakin jiyya na solvothermal yana haɓaka cikakkiyar hydrolysis na precursor na aluminium kuma yana samar da ƙungiyoyin aluminium hydroxyl masu ƙunshe da wani yanki. Idan aka kwatanta da MA da aka shirya ta hanyar EISA na gargajiya, OMA da aka shirya ta hanyar SA-EISA yana da ƙarar pore mafi girma, ingantaccen yanki na musamman da ingantaccen yanayin zafi. A nan gaba, ana iya amfani da hanyar EISA don shirya babban buɗaɗɗen buɗaɗɗen MA tare da ƙimar juzu'i mai girma da ingantaccen zaɓi ba tare da amfani da wakilin reaming ba.
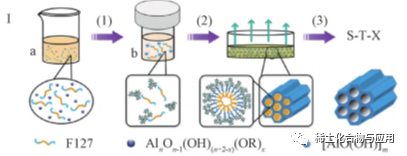
Hoto na 1 mai gudana na hanyar SA-EISA don haɗa kayan OMA
1.2.2 sauran matakai
Shirye-shiryen MA na al'ada yana buƙatar daidaitaccen sarrafa sigogin kira don cimma ingantaccen tsarin mesoporous, kuma cire kayan samfuri kuma yana da ƙalubale, wanda ke rikitar da tsarin haɗin. A halin yanzu, wallafe-wallafe da yawa sun ba da rahoton haɗakar MA tare da samfuri daban-daban. A cikin 'yan shekarun nan, binciken ya fi mayar da hankali kan haɗakar MA tare da glucose, sucrose da sitaci a matsayin samfurori ta aluminum isopropoxide a cikin ruwa mai ruwa.Mafi yawan waɗannan kayan MA an haɗa su daga aluminum nitrate, sulfate da alkoxide a matsayin tushen aluminum. Hakanan ana samun MA CTAB ta hanyar gyara kai tsaye na PB azaman tushen aluminum. MA tare da kaddarorin tsarin daban-daban, watau Al2O3) -1, Al2O3) -2 da al2o3Kuma yana da kwanciyar hankali mai kyau na thermal. Bugu da kari na surfactant baya canza dabi'ar crystal tsarin PB, amma canza stacking yanayin barbashi. Bugu da kari, samuwar Al2O3-3 an kafa ta da mannewa na nanoparticles stabilized ta Organic sauran ƙarfi PEG ko aggregation a kusa da PEG. Koyaya, rarraba girman pore na Al2O3-1 yana da kunkuntar sosai. Bugu da ƙari, an shirya masu samar da kayan aiki na palladium tare da MA na roba.
A karon farko, an shirya MA tare da kunkuntar kunkuntar girman girman rabe-raben ta amfani da arha da arziƙin aluminium baƙar fata ABD. Tsarin samarwa ya haɗa da tsarin cirewa a ƙananan zafin jiki da matsa lamba na al'ada. Daskararrun barbashi da aka bari a cikin tsarin hakar ba za su ƙazantar da muhalli ba, kuma ana iya tara su da ƙananan haɗari ko sake amfani da su azaman filler ko tara a cikin kankare aikace-aikace. Ƙayyadaddun yanki na haɗin MA shine 123 ~ 162m2 / g, Rarraba girman raƙuman ruwa yana kunkuntar, radius mafi girma shine 5.3nm, kuma porosity shine 0.37 cm3 / g. Kayan yana da girman nano kuma girman crystal kusan 11nm. Ƙaƙƙarfan tsarin jiha sabon tsari ne don haɗa MA, wanda za'a iya amfani dashi don samar da abin sha na radiochemical don amfanin asibiti. Aluminum chloride, ammonium carbonate da glucose albarkatun kasa suna gauraye a cikin wani molar rabo na 1: 1.5: 1.5, da kuma MA da aka hada da wani sabon m-jihar mechanochemical dauki.By mayar da hankali131I a thermal baturi kayan aiki, da jimlar yawan amfanin ƙasa na131I bayan maida hankali ne 90%, da kuma samu131I / TB maida hankali ne a high radio. fahimtar amfani da babban kashi 131I [NaI] capsules don maganin ciwon daji na thyroid.
Don taƙaitawa, a nan gaba, ana iya haɓaka ƙananan samfuran ƙwayoyin ƙwayoyin cuta don gina manyan matakan da aka ba da umarnin pore, yadda ya kamata daidaita tsarin, ilimin halittar jiki da kaddarorin sinadarai na kayan, da kuma haifar da babban yanki da kuma ba da umarnin wormhole MA. Bincika samfura masu arha da maɓuɓɓugar aluminum, inganta tsarin haɓakawa, fayyace hanyar haɗin gwiwa da jagorar tsari.
Hanyar gyaggyarawa na 2 MA
Hanyoyin rarraba kayan aiki iri ɗaya akan mai ɗauka na MA sun haɗa da impregnation, in-situ synthe-sis, hazo, musanya ion, gaurayawan inji da narkewa, daga cikinsu na farko sune aka fi amfani da su.
2.1 in-wurin kira Hanyar
Ƙungiyoyin da aka yi amfani da su a cikin gyare-gyaren aiki suna ƙarawa a cikin tsarin shirya MA don gyarawa da daidaita tsarin kwarangwal na kayan da inganta aikin haɓakawa. Ana nuna tsarin a cikin Hoto 2. Liu et al. Ni/Mo-Al2O3in da aka haɗa tare da P123 azaman samfuri. Dukansu Ni da Mo an tarwatsa su cikin tashoshi na MA da aka ba da oda, ba tare da lalata tsarin MA ba, kuma a fili an inganta aikin haɓaka. Ɗauki hanyar haɓaka cikin-wuri akan haɗaɗɗen gamma-al2o3substrate, Idan aka kwatanta da γ-Al2O3, MnO2-Al2O3 yana da ƙayyadaddun yanki na BET mafi girma da ƙarar pore, kuma yana da tsarin mesoporous na bimodal tare da kunkuntar girman girman pore. MnO2-Al2O3 yana da saurin adsorption kudi da babban inganci don F-, kuma yana da kewayon aikace-aikacen pH (pH = 4 ~ 10), wanda ya dace da yanayin aikace-aikacen masana'antu masu amfani. Ayyukan sake amfani da MnO2-Al2O3 ya fi na γ-Al2O.Tsarin kwanciyar hankali yana buƙatar ƙara inganta shi. Don taƙaitawa, MA gyare-gyaren kayan da aka samu ta hanyar haɗin ginin yana da kyakkyawan tsari na tsari, hulɗa mai ƙarfi tsakanin ƙungiyoyi da masu ɗaukar alumina, haɗuwa mai ƙarfi, babban nauyin kayan aiki, kuma ba su da sauƙi don haifar da zubar da kayan aiki masu aiki a cikin tsarin amsawar catalytic, kuma aikin catalytic yana inganta sosai.
Hoto 2 Shiri na aiki MA ta hanyar haɗin-wuri
2.2 Hanyar impregnation
Yin nutsewa da shirye-shiryen MA a cikin ƙungiyar da aka gyara, da kuma samun kayan aikin MA da aka gyara bayan jiyya, don gane tasirin catalysis, adsorption da makamantansu. Kai et al. an shirya MA daga P123 ta hanyar sol-gel, kuma an jika shi a cikin ethanol da maganin tetraethylenepentamine don samun kayan MA da aka gyara na amino tare da aikin adsorption mai ƙarfi. Bugu da kari, Belkacemi et al. tsoma a cikin ZnCl2solution ta hanyar wannan tsari don samun oda zinc doped modified MA kayan.The musamman surface area da pore girma ne 394m2 / g da 0.55 cm3 / g, bi da bi. Idan aka kwatanta da in-situ kira hanya, da impregnation hanya yana da mafi kyau kashi watsawa, barga mesoporous tsarin da kuma mai kyau adsorption yi, amma da hulda da karfi tsakanin aiki aka gyara da kuma alumina m ne mai rauni, da catalytic aiki ne sauƙi tsoma baki ta waje dalilai.
3 ci gaban aiki
Haɗin duniyar MA tare da kaddarorin musamman shine yanayin ci gaba a nan gaba. A halin yanzu, akwai hanyoyin haɗin kai da yawa. Siffofin tsari suna shafar aikin MA. Ƙayyadadden yanki, ƙarar pore da diamita na MA za a iya daidaita shi ta nau'in samfuri da abun da ke gaba na aluminum. Matsakaicin ƙididdiga da ƙididdiga na samfuri na polymer suna shafar takamaiman yanki da ƙarar pore na MA. Suzuki da Yamauchi gano cewa calcination zafin jiki da aka ƙara daga 500 ℃ zuwa 900 ℃. The budewa za a iya ƙara da surface area za a iya rage. Bugu da kari, da rare duniya gyare-gyaren magani inganta aiki, saman thermal kwanciyar hankali, tsarin da kwanciyar hankali da kuma surface acidity na MA kayan a cikin catalytic tsari, da kuma saduwa da ci gaban MA aiki.
3.1 Defluorination Adsorbent
Fluorine a cikin ruwan sha a kasar Sin yana da matukar illa. Bugu da kari, da karuwa da fluorine abun ciki a masana'antu zinc sulfate bayani zai kai ga lalata na lantarki farantin, da tabarbarewar yanayin aiki, da koma bayan da ingancin lantarki zinc da rage yawan sake fa'ida ruwa a cikin acid yin tsarin da electrolysis tsari na fluidized gado makera gasa flue gas. A halin yanzu, hanyar adsorption ita ce mafi ban sha'awa a cikin hanyoyin da aka saba amfani da su na rigar defluorination. Duk da haka, akwai wasu gazawa, irin su rashin iyawar talla, kunkuntar pH da ke samuwa, gurɓataccen gurɓataccen abu da sauransu. Kunna carbon, amorphous alumina, kunna alumina da sauran adsorbents an yi amfani da defluorination na ruwa, amma farashin adsorbents ne high, da kuma adsorption iya aiki na F-a tsaka tsaki bayani ko high maida hankali ne low.Activated alumina ya zama mafi yadu nazarin adsorbent ga fluoride kau saboda ta high kusanci da kuma zažužžukan zuwa fluoride shi ne iyakacin iyaka. iya aiki na fluoride, kuma kawai a pH <6 zai iya samun mai kyau fluoride adsorption yi.MA ya janyo hankalin m da hankali a cikin muhalli gurbatawa kula saboda da babban takamaiman surface area, musamman pore size sakamako, acid-tushe yi, thermal da kuma inji kwanciyar hankali. Kundu et al. shirye-shiryen MA tare da matsakaicin ƙarfin adsorption na fluorine na 62.5 mg/g. Ƙarfin adsorption na fluorine na MA yana da tasiri sosai ta hanyar halayen tsarinsa, irin su takamaiman yanki, ƙungiyoyi masu aiki, girman pore da jimlar pore. Daidaita tsarin da aikin MA shine hanya mai mahimmanci don inganta aikin adsorption.
Saboda hard acid na La da kuma ƙaƙƙarfan asali na fluorine, akwai ƙaƙƙarfan alaƙa tsakanin La da ions fluorine. A cikin 'yan shekarun nan, wasu binciken sun gano cewa La a matsayin mai gyara zai iya inganta ƙarfin tallan fluoride. Duk da haka, saboda ƙarancin tsarin tsarin adsorbents na ƙasa, mafi ƙarancin ƙasa suna shiga cikin maganin, yana haifar da gurɓataccen ruwa na biyu da cutarwa ga lafiyar ɗan adam. A daya bangaren kuma, yawan sinadarin aluminium a muhallin ruwa na daya daga cikin guba ga lafiyar dan Adam. Sabili da haka, ya zama dole don shirya nau'in adsorbent mai hade tare da kwanciyar hankali mai kyau kuma babu leaching ko žasa leaching na wasu abubuwa a cikin tsarin cire fluorine. MA gyara ta La da Ce aka shirya ta hanyar impregnation (La/MA da Ce/MA). rare duniya oxides aka samu nasarar ɗora Kwatancen a kan MA surface a karon farko, wanda yana da mafi girma defluorination performance.The main hanyoyin da fluorine kau ne electrostatic adsorption da sinadarai adsorption, da electron janyo hankalin surface tabbatacce cajin da ligand musayar dauki hadawa da surface hydroxyl, da hydroxyl aiki kungiyar a kan adsorbent surface haifar da hydrogen bond tare da F-, da ikon haɓakawa na adsorption na Lasor. ƙarin wuraren adsorption na hydroxyl, kuma ƙarfin tallan F yana cikin tsari na La/MA>Ce/MA>MA. Tare da haɓakar ƙaddamarwa na farko, ƙarfin tallan furotin yana ƙaruwa. Sakamakon adsorption shine mafi kyau lokacin da pH ya kasance 5 ~ 9, da kuma tsarin tallan furotin na fluorine tare da Langmuir isothermal adsorption model. Bugu da ƙari, ƙazantattun ions sulfate a cikin alumina na iya tasiri sosai ga ingancin samfurori. Ko da yake an gudanar da binciken da ya danganci alumina da aka gyara a duniya, yawancin binciken yana mayar da hankali kan tsarin adsorbent, wanda ke da wuya a yi amfani da shi a masana'antu. A nan gaba, za mu iya nazarin tsarin rarrabawar fluorine a cikin maganin zinc sulfate da kuma halayen ƙaura na ions fluorine, samun ingantaccen, ƙananan farashi da sabuntawa na fluorine ion sulfortgynt a cikin maganin zinc ion sulfotell. tsarin, da kuma kafa tsarin sarrafa tsari don magance babban maganin fluorine dangane da ƙarancin duniya MA nano adsorbent.
3.2 Mai kara kuzari
3.2.1 Busassun sake fasalin methane
Rare ƙasa na iya daidaita acidity (basicity) na porous kayan, ƙara oxygen vacances, da kuma hada catalysts tare da uniform watsawa, nanometer sikelin da kwanciyar hankali. Ana amfani da shi sau da yawa don tallafawa karafa masu daraja da karafa na mika mulki don haifar da methanation na CO2. A halin yanzu, rare ƙasa modified mesoporous kayan suna tasowa zuwa methane bushe gyara (MDR), photocatalytic ƙasƙanci na VOCs da wutsiya gas tsarkakewa.Compared tare da daraja karafa (kamar Pd, Ru, Rh, da dai sauransu) da sauran mika mulki karafa (kamar Co, Fe, da dai sauransu), Ni / Al2O3catalyst da low cost da high amfani da high selective aiki da low cost da high selectively aiki da low cost da kuma high cost da high selectively domin ta high cost da kuma high cost. methane. Duk da haka, ɓacin rai da ajiyar carbon na Ni nanoparticles a saman Ni/Al2O3 yana kaiwa ga saurin kashe mai kara kuzari. Saboda haka, wajibi ne don ƙara hanzari, gyara mai ɗaukar hoto da inganta hanyar shirye-shirye don inganta aikin catalytic, kwanciyar hankali da juriya. Gabaɗaya, ƙananan oxides na ƙasa za a iya amfani da su azaman masu haɓakawa na tsari da lantarki a cikin abubuwan haɓakawa daban-daban, kuma CeO2 yana haɓaka tarwatsawar Ni kuma yana canza kaddarorin ƙarfe Ni ta hanyar hulɗar tallafin ƙarfe mai ƙarfi.
MA ana amfani dashi ko'ina don haɓaka rarrabuwar karafa, da samar da kamewa ga karafa masu aiki don hana haɓakar su. La2O3 tare da babban ƙarfin ajiyar iskar oxygen yana haɓaka juriya na carbon a cikin tsarin juyawa, kuma La2O3 yana haɓaka watsawar Co akan mesoporous alumina, wanda ke da babban aikin gyare-gyare da juriya. La2O3promoter yana ƙara yawan aikin MDR na Co/MA mai haɓakawa, kuma Co3O4and CoAl2O4phases an kafa su a kan farfajiyar mai kara kuzari.Duk da haka, La2O3 da aka tarwatsa yana da ƙananan hatsi na 8nm ~ 10nm. A cikin tsarin MDR, hulɗar cikin-wuri tsakanin La2O3 da CO2 da aka samar da La2O2CO3mesophase, wanda ya haifar da kawar da CxHy mai tasiri a kan mai kara kuzari. La2O3 yana haɓaka raguwar hydrogen ta hanyar samar da mafi girman yawan electron da haɓaka sarari oxygen a cikin 10% Co/MA. Ƙarin La2O3 yana rage alamar kunnawa na amfani da CH4. Sabili da haka, Ƙididdigar juyawa na CH4 ya karu zuwa 93.7% a 1073K K. Bugu da ƙari na La2O3 ya inganta aikin catalytic, ya inganta rage H2, ya karu yawan wuraren aiki na Co0, ya haifar da ƙarancin ajiyar carbon kuma ya kara yawan iskar oxygen zuwa 73.3%.
An goyan bayan Ce da Pr akan Ni/Al2O3catalyst ta hanyar daidaitaccen girman girman ciki a Li Xiaofeng. Bayan ƙara Ce da Pr, zaɓin zuwa H2 ya ƙaru kuma zaɓin zuwa CO ya ragu. MDR da Pr ya gyara yana da kyakkyawan ƙarfin kuzari, kuma zaɓin zuwa H2 ya karu daga 64.5% zuwa 75.6%, yayin da zaɓin zuwa CO ya ragu daga 31.4% Peng Shujing et al. Ana amfani da hanyar sol-gel, Ce-gyara MA an shirya shi tare da aluminum isopropoxide, isopropanol ƙarfi da cerium nitrate hexahydrate. An ƙara ƙayyadaddun yanki na samfurin. Bugu da kari na Ce rage tari na sanda-kamar nanoparticles a kan MA surface. Wasu rukunin hydroxyl a saman γ- Al2O3 an rufe su da mahallin Ce. An inganta kwanciyar hankali na thermal na MA, kuma babu wani canjin lokaci na crystal ya faru bayan calcination a 1000 ℃ na 10 hours. Wang Baowei et al. Shirye-shiryen MA abu CeO2-Al2O4by hanyar daidaitawa. An tarwatsa CeO2 tare da ƙananan ƙananan hatsi iri ɗaya a cikin alumina. Bayan tallafawa Co da Mo akan CeO2-Al2O4, hulɗar tsakanin alumina da kayan aiki Co da Mo an hana shi yadda ya kamata ta CEO2
Masu tallata ƙasa da ba kasafai ba (La, Ce, y da Sm) suna Haɗe tare da Co/MA mai haɓakawa don MDR, kuma ana nuna tsarin a fig. 3. da rare ƙasa talla iya inganta watsawa na Co a kan MA m da kuma hana agglomeration na co barbashi. ƙarami girman barbashi, da karfi da Co-MA hulda, da karfi da catalytic da sintering ikon a YCo / MA kara kuzari, da kuma m sakamakon da dama masu talla a kan MDR aiki da carbon deposition.Fig. 4 shine HRTEM iMAge bayan maganin MDR a 1023K, Co2: ch4: N2 = 1 ∶ 1 ∶ 3.1 na awanni 8. Co barbashi wanzu a cikin nau'i na baki spots, yayin da MA dako ya wanzu a cikin nau'i na launin toka, wanda ya dogara da bambanci na electron yawa. a cikin hoton HRTEM tare da 10% Co / MA (fig. 4b), ana lura da haɓakar ƙwayoyin ƙarfe na Co karfe akan ma masu ɗaukar nauyi. YCo/MA yana da ma'amalar Co-MA mai ƙarfi, kuma aikin sa ya fi sauran masu kara kuzari. ƙari, kamar yadda aka nuna a cikin ɓaure. 4b zuwa 4f, ana samar da hollow carbon nanowires (CNF) akan masu kara kuzari, waɗanda ke hulɗa da kwararar iskar gas kuma suna hana mai haɓakawa daga kashewa.

Hoto 3 Tasirin ƙarar ƙasa da ba kasafai ba akan kaddarorin jiki da sinadarai da aikin MDR mai haɓakawa na Co/MA mai haɓakawa
3.2.2 Deoxidation mai kara kuzari
Fe2O3/Meso-CeAl, Ce-doped Fe-based deoxidation catalyst, an shirya shi ta hanyar oxidative dehydrogenation na 1-butene tare da CO2as mai laushi mai laushi, kuma an yi amfani dashi a cikin kira na 1,3-butadiene (BD). Ce da aka sosai tarwatsa a alumina matrix, kuma Fe2O3 / meso aka sosai tarwatsaFe2O3 / Meso-CeAl-100 kara kuzari ba kawai yana da sosai tarwatsa baƙin ƙarfe jinsunan da kyau tsarin Properties, amma kuma yana da kyau oxygen ajiya iya aiki, don haka yana da kyau adsorption da kunnawa damar CO2. Kamar yadda aka nuna a cikin Hoto 5, Hotunan TEM sun nuna cewa Fe2O3 / Meso-CeAl-100 ne na yau da kullumYa nuna cewa tsarin tashar tashar tsutsa mai kama da MesoCeAl-100 yana da sako-sako da porous, wanda ke da amfani ga rarraba kayan aiki masu aiki, yayin da aka tarwatsa Ce sosai an samu nasarar doped a cikin alumina matrix. The daraja karfe mai kara kuzari kayan shafa gamuwa matsananci-ƙananan watsi misali na motoci ya ɓullo da pore tsarin, mai kyau hydrothermal kwanciyar hankali da kuma babban oxygen ajiya iya aiki.
3.2.3 Mai kara kuzari ga Motoci
Pd-Rh yana goyan bayan quaternary aluminum-based rare earth complexes AlCeZrTiOx da AlLaZrTiOx don samun kayan shafa mai kara kuzari. Mesoporous aluminum-based rare earth complex Pd-Rh/ALC za a iya samu nasarar amfani da shi azaman CNG abin hawa shaye tsarkakewa mai kara kuzari tare da mai kyau karko, da kuma juzu'i yadda ya dace na CH4, babban bangaren CNG abin hawa shaye gas, ne kamar yadda 97.8%. Ɗauki hanyar mataki ɗaya na hydrotherMAl don shirya waccan ƙarancin ƙasa mai haɗaɗɗun kayan don gane haɗin kai, An yi umarni da precursors mesoporous tare da yanayin metastable da haɓakar haɓaka, kuma haɗin RE-Al ya dace da ƙirar “haɗin haɓakar haɓaka”, don haka fahimtar tsarkakewar mota-haɗe-haɗe-haɗe-haɗe-haɗe.
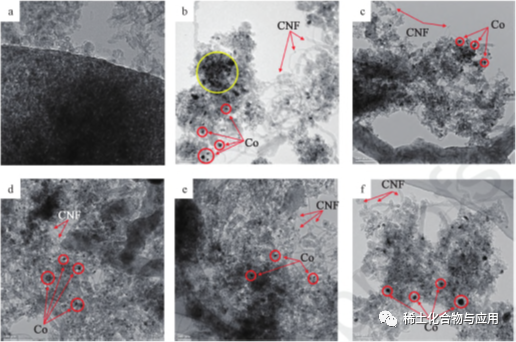
Hoto 4 HRTEM Hotunan ma (a), Co/MA (b), LaCo/MA(c), CeCo/MA (d), YCo/MA (e) da SmCo/MA (f)
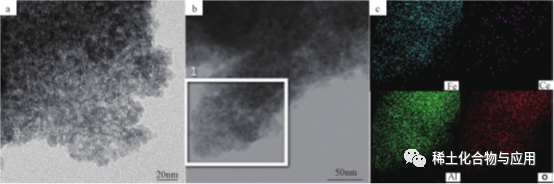
Hoto 5 Hoton TEM (A) da zane-zane na EDS (b,c) na Fe2O3/Meso-CeAl-100
3.3 aiki mai haske
Electrons na abubuwan da ba kasafai ba na duniya suna cikin sauƙi don canzawa tsakanin matakan makamashi daban-daban kuma suna fitar da haske. Ana amfani da ions da ba kasafai ba sau da yawa azaman masu kunnawa don shirya kayan haske. Rare ƙasa ions za a iya ɗora Kwatancen a saman aluminum phosphate m microspheres ta hanyar kwafi da ion musayar hanya, da luminescent kayan AlPO4∶RE (La, Ce, Pr, Nd) za a iya shirya. A luminescent raƙuman ruwa ne a cikin kusa ultraviolet region.MA aka sanya a cikin bakin ciki fina-finai saboda da inertia, low dielectric akai-akai da kuma low conductivity, wanda ya sa shi zartar da lantarki da Tantancewar na'urorin, bakin ciki fina-finai, shingen, na'urori masu auna sigina, da dai sauransu Har ila yau, ana iya amfani da ji amsa daya-girma photonic lu'ulu'u, makamashi shafi da anti-reflection. Wadannan na'urori an tattara fina-finai tare da takamaiman tsayin hanya na gani, don haka ya zama dole don sarrafa ma'auni na refractive da kauri. A halin yanzu, titanium dioxide da zirconium oxide tare da babban ma'auni mai mahimmanci da silicon dioxide tare da ƙananan ƙididdiga suna amfani da su don tsarawa da gina irin waɗannan na'urori. An fadada kewayon samuwa na kayan da ke da kaddarorin sinadarai daban-daban, wanda ke ba da damar tsara na'urorin firikwensin photon na ci gaba. Gabatarwar fina-finai na MA da oxyhydroxide a cikin ƙirar na'urori na gani yana nuna babban yuwuwar saboda ma'anar refractive yayi kama da na silicon dioxide.Amma abubuwan sinadaran sun bambanta.
3.4 thermal kwanciyar hankali
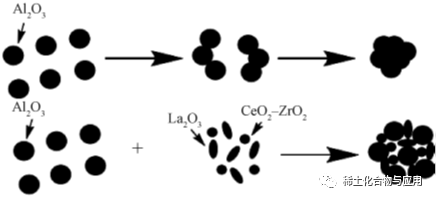
Tare da haɓakar zafin jiki, sintering da gaske yana rinjayar tasirin amfani da mai kara kuzari na MA, kuma takamaiman yanki yana raguwa kuma γ-Al2O3in crystalline lokaci yana canzawa zuwa δ da θ zuwa χ matakai. Kayayyakin ƙasa maras tsada suna da kyakkyawar kwanciyar hankali na sinadarai da kwanciyar hankali mai zafi, babban daidaitawa, da sauƙin samuwa da albarkatun ƙasa. Bugu da kari na rare ƙasa abubuwa iya inganta thermal kwanciyar hankali, high zafin jiki hadawan abu da iskar shaka juriya da inji Properties na m, da kuma daidaita surface acidity na carrier.La da Ce ne da aka fi amfani da kuma nazarin gyare-gyare abubuwa. Lu Weiguang da sauransu sun gano cewa ƙari na abubuwan da ba kasafai ba na duniya ya hana yaduwar ƙwayoyin alumina yadda ya kamata, La da Ce sun kare ƙungiyoyin hydroxyl a saman alumina, sun hana sintering da canjin lokaci, kuma sun rage lalacewar babban zafin jiki zuwa tsarin mesoporous. Alumina da aka shirya har yanzu yana da takamaiman yanki na musamman da ƙarar pore. Duk da haka, da yawa ko ƙarancin ƙarancin ƙasa zai rage kwanciyar hankali na alumina. Li Yanqiu et al. ya kara 5% La2O3to γ-Al2O3, wanda ya inganta kwanciyar hankali na thermal kuma ya kara girman pore da takamaiman yanki na mai ɗaukar alumina. Kamar yadda ake iya gani daga Hoto na 6, La2O3daɗawa zuwa γ-Al2O3, Ingantacciyar kwanciyar hankali na mai ɗaukar abubuwa na ƙasa da ba kasafai ba.
A cikin aiwatar da doping nano-fibrous barbashi tare da La zuwa MA, BET surface area da pore girma na MA-La sun fi na MA lokacin da zafi jiyya zafin jiki ya karu, kuma doping tare da La yana da fili retarding sakamako a sintering a high zafin jiki. kamar yadda aka nuna a fig. 7, tare da karuwar zafin jiki, La yana hana haɓakar haɓakar hatsi da canjin lokaci, yayin da figs. 7a da 7c suna nuna tarin nano-fibrous barbashi. a cikin fig. 7b, diamita na manyan barbashi samar da calcination a 1200 ℃ ne game da 100nm.It alama da gagarumin sintering na MA. Bugu da ƙari, idan aka kwatanta da MA-1200, MA-La-1200 ba ya haɗuwa bayan maganin zafi. Tare da ƙari na La, barbashi na nano-fiber suna da mafi kyawun iya yin sintiri. ko da a mafi girma calcination zafin jiki, doped La har yanzu sosai tarwatsa a kan MA surface. Ana iya amfani da La modified MA azaman mai ɗaukar Pd mai haɓakawa a cikin amsawar C3H8oxidation.
Hoto 6 Tsarin tsarin tsarin alumina tare da kuma ba tare da ƙarancin abubuwan duniya ba

Hoto na 7 TEM Hotuna na MA-400 (a), MA-1200 (b), MA-La-400 (c) da MA-La-1200 (d)
4 Kammalawa
An gabatar da ci gaban shirye-shirye da aikace-aikacen aikace-aikacen kayan aikin MA da ba kasafai ba. Rare earth modified MA ana amfani da ko'ina. Kodayake an gudanar da bincike mai yawa a cikin aikace-aikacen catalytic, kwanciyar hankali na thermal da adsorption, yawancin kayan suna da tsada mai tsada, ƙananan adadin doping, tsari mara kyau kuma yana da wuyar zama masana'antu. Ana buƙatar yin aikin da ke gaba a nan gaba: haɓaka abun da ke ciki da tsarin da ba kasafai aka gyara ba MA, zaɓi tsarin da ya dace, Haɗu da haɓaka aikin; Ƙaddamar da samfurin sarrafa tsari bisa tsarin aiki don rage farashin da kuma gane samar da masana'antu; Don haɓaka fa'idodin albarkatun ƙasa na kasar Sin, ya kamata mu bincika tsarin da ba kasafai ake yin gyare-gyaren duniya ba, da inganta ka'idar da tsarin shirya sauye-sauyen duniya da ba kasafai ba.
Ayyukan Asusun: Shaanxi Kimiyya da Fasaha Gabaɗaya Aikin Ƙirƙirar Ƙirƙirar (2011KTDZ01-04-01); Lardin Shaanxi 2019 Aikin Bincike na Musamman na Kimiyya (19JK0490); 2020 aikin bincike na musamman na kimiyya na Kwalejin Huaqing, Jami'ar Gine-gine da Fasaha ta Xi (20KY02)
Source: Rare Duniya
Lokacin aikawa: Jul-04-2022